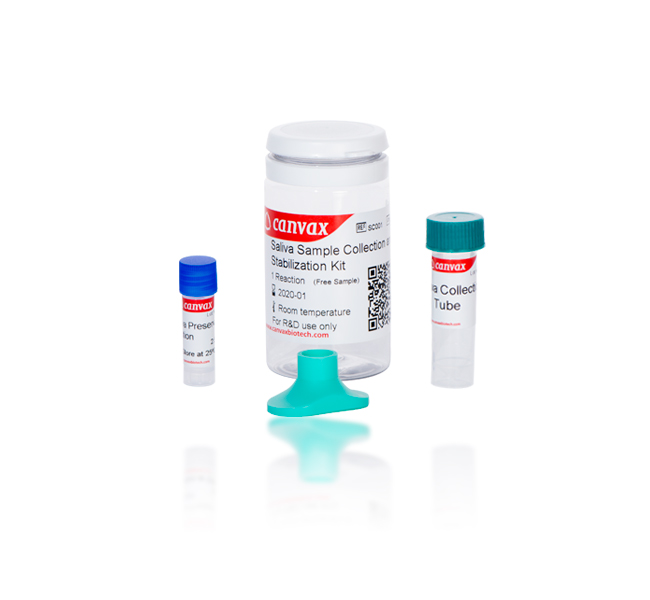
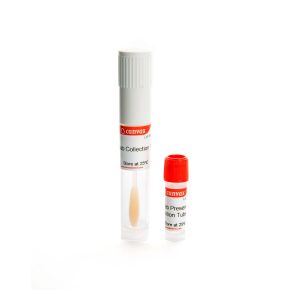
CVX™ Virus Collection & Processing Kit
For collection and transportation of Saliva specimens containing Viruses from prior Direct PCR
Saliva is a promising sample for expanding and facilitating testing due to the ease, safety, and non- invasive nature of its collection and its relatively high viral load. CVX™ Virus Collection & Processing Kit is designed to collect, and transport saliva specimens containing viruses from patients with signs and symptoms of respiratory infection and subsequent sample processing prior Direct PCR. Saliva sample are collected by spitting inside the collection funnel which has been assembled with the collection tube. After collecting ≈1 mL of Saliva, the collection tube is sent to the laboratory for sample processing. You can store collected Saliva at room temperature for no more than 7 days before processing without adding buffers for the stabilization of nucleic acid viral.
Researchers at Yale University developed a saliva-based method for the detection of SARS-CoV-2. The method uses proteinase K, followed by a heat step to make viral RNA detectable in a saliva sample instead of using kits to extract the RNA. It includes the PK solution (containing proteinase K) that has been specifically formulated to be used following the open-source protocol developed by Yale’s researchers and obtain PCR-ready nucleic acids from Saliva.
Price: 0.00 €